The fish processing industry produces more than 60% by-products as waste, which incorporates head, skin, trimmings, fins, frames, viscera and roes. These by-product wastes contain good amount of protein rich material that are processed into make aquafeed.

Master Student
Tamil Nadu Dr. J. Jayalaithaa Fisheries University

Assistant Professor
Tamil Nadu Dr. J. Jayalaithaa Fisheries University

Master Student
Tamil Nadu Dr. J. Jayalaithaa Fisheries University
INTRODUCTION
Aquaculture plays a major role in the food production industry and supporting the protein needs of world population. Aquaculture is reliant primarily on fishmeal from wild fish as its main source of protein. Many researches being done to form alternative protein sources and improving the utilization efficiency of dietary protein is important for the sustainable development of aquaculture. The fish processing industry produces more than 60% by-products as waste, which incorporates head, skin, trimmings, fins, frames, viscera and roes. For, this reason better waste management system required overcoming the environmental issues and it should have economical value at the same time. These by-product wastes contain good amount of protein rich material that are processed into make aquafeed. Protein hydrolysates are breakdown products of enzymatic conversion of proteins into smaller peptides that contain 2–20 amino acids. Due to the smaller peptide size, hydrolysates are the foremost available amino acid source for various physiological functions of animal body.
SOURCES OF FPH
Fish protein hydrolysate (FPH) can be produced from both freshwater and marine fish viscera. Fish fillets are often considered the most desired product in the market, even up to 60% of the harvested fish volume being discarded during processing. The production of FPH also uses fish skin from the fish processing sector, which is rich in collagen and gelatine. A substantial amount of protein is also found in fish heads produced by the fish processing industry. FPH can be also produced from fish bones and fish frames of various species including shortfin scad, cod and ribbon fish.
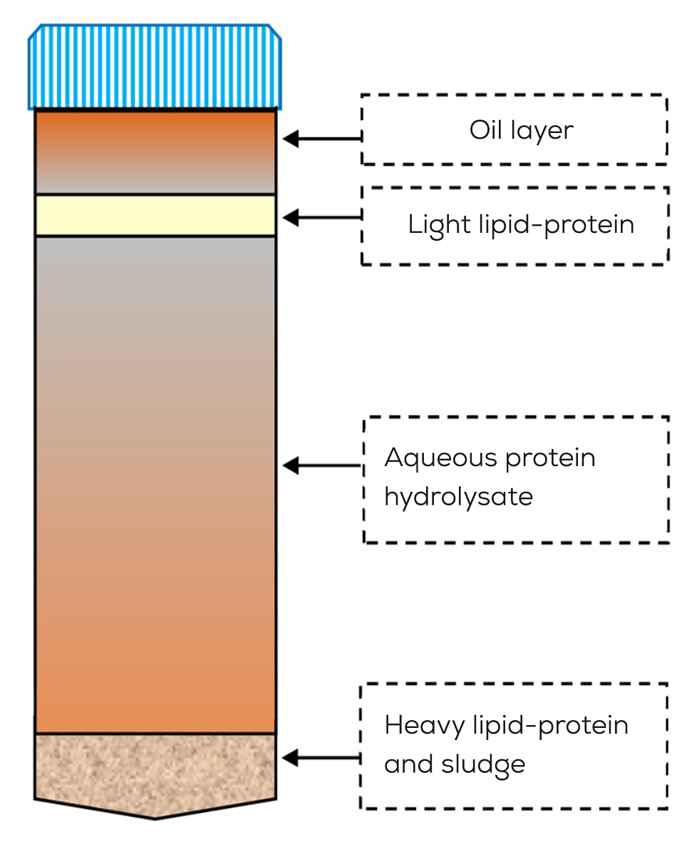
PRODUCTION OF FPH
There are four methods that can be used to prepare protein hydrolysates from fish processing waste: enzymatic, acid, alkali and microbial fermentation. The most popular techniques among these are enzymatic hydrolysis and chemical hydrolysis (acid and alkali hydrolysis). Even though the chemical hydrolysis method is yields a large amount of recovered protein, it lacks some essential amino acids. The action of endogenous digestive enzymes in the fish controls the autolysis process used to make FPH. In bacterial fermentation the growth of lactic acid bacteria which produce acid and antimicrobial substances which inhibit competing bacteria but the removal of lipid is not possible.
ENZYMATIC HYDROLYSIS
Enzymatic hydrolysis of proteins is the most frequently opted and most promising compared to other processes as it results in products with high nutritive value and intestinal absorption characteristics. Further, this method requires comparatively small quantity of enzymes at mild hydrolytic condition that can be deactivated easily. The use of enzymes does not destroy amino acids and resulting mixtures of peptides can be easily purified. Proteolytic enzymes are commercially available from several sources like animal, plant and microbes. Common enzymes used for FPH production are pepsins, papain, bromelain, alcalase, neutrase and flavourzyme. Enzymes of microbial origin are considered as having greater pH and temperature stability.
ACID HYDROLYSIS
The complete fish protein hydrolysis using hydrochloric acid or sulfuric acid has been performed at elevated temperatures and also at high pressures. The solution is neutralised to pH 6.0 to 7.0 after hydrolysis, condensed and dried further. Absolute hydrolysis process can be accomplished in 18 hours at 118 °C in 6 M HCl. Due to the low cost of processing and the consequent widespread hydrolysis, this method is often used to convert secondary raw material from fish into fertilizer (Elavarasan et al., 2019). However, during acid hydrolysis important amino acids such as tryptophan, methionine, cystine are normally lost. In addition, asparagine and glutamine are converted into aspartic acid and glutamic acid respectively. Due to the formation of salts during the neutralization process, acquired hydrolysates have weak functional properties. Therefore, many methods of separation of salt have been suggested, such as nano filtration and the use of ion exchange resins with excellent performance.
ALKALINE HYDROLYSIS
Wide water-soluble polypeptides are easily broken in the alkaline hydrolysis process. Sodium hydroxide is mainly used in the alkali hydrolysis process. A high pH of 12.5 at 95 °C for 20 minutes was used in the small-scale batch system developed to hydrolyze the fish protein concentrates to increase functionality (Elavarasan et al., 2019). The key drawback of this process is the development of low amino acid content in hydrolysates such as cystine, lysine, arginine, serine, threonine, isoleucine and produce residues such as lanthionine and lysinoalanine.
FERMENTATION PROCESS
It uses natural or cultured microorganisms (starter cultures). During the growth of the organism, they hydrolyze the nutrients particularly carbohydrate and proteins. The extracellular microbial proteases hydrolyze the proteins into peptides of varied molecular size. Depend upon the microorganism species used for fermentation, the property and quality of protein hydrolysate differs.
![]() NUTRITIONAL PROPERTIES
NUTRITIONAL PROPERTIES
The protein contents of FPH vary between 60.0% and 90.0% depending on the types and sources of raw material and hydrolysis protocol (Kristinsson & Rasco, 2000; Bhaskar et al., 2008). Due to the solubilisation of protein during enzymatic hydrolysis and removal of lipid after hydrolysis increase the protein content of FPH. However, the protein content of the FPH also varies with the temperature during drying process. The raw materials having higher percentage of lipids that produce lower amount of solubilized protein. Reduced lipid content in FPH may increase the stability of the final product towards lipid oxidation, which may increase the shelf life of FPH in storage condition. The high ash contents in FPH may be due to the addition of alkali for pH adjustment and breakdown of bones in the raw material. The moisture content of FPH should be below 10% of total composition to retain its quality (Bhaskar et al. 2008; Chalamaiah et al. 2010).
FUNCTIONAL PROPERTIES
Solubility
Protein solubility is referred to as the quantity of protein that goes into the solution under identified settings. Hydrophobic interactions promote protein-protein interactions which decreases their solubility whereas ionic interactions support protein water interactions favoring the solubility characteristics. The potential applications of proteins in FPH can be expanded with higher solubility. The level of degree hydrolysis (DH), pH, temperature, ionic strength, type of solvent and processing conditions strongly influence the solubility of protein. Long time hydrolysis leading to a high degree of hydrolysis resulting in protein solutions with smaller molecular weights of higher solubility.
Emulsifying properties
Emulsions are thermodynamically unstable systems as a result of the large positive energy at the interface of the two liquids. Fish protein hydrolysates, on account of their enhanced amphiphilic nature, act as good emulsifier. Protein hydrolysis results in exposure of more hydrophilic and hydrophobic groups facilitating their orientation at the oil–water interface enabling effective adsorption. FPH with a higher DH, a higher amount of smaller peptides are formed, which may result in a drastic loss of emulsifying properties. Therefore, a low DH along with a careful choice of enzymes is a key aspect if enhanced emulsifying properties are desired.
![]() Foaming properties
Foaming properties
Similar to emulsifying properties, foaming properties also rely on protein’s surface properties. Hydrophobicity has a significant correlation to foaming formation and comparable to other functional properties, these attributes are also related to the degree of hydrolysis. Foaming properties are usually expressed as foaming capacity and foam stability. Foaming capacity is referred to as the ability of protein to form foams also described as over run, which is the excess amount of foam produced on whipping a protein solution in comparison to the initial protein solution volume. Foam stability is measured as the decrease in volume of the whipped protein solution within a specific period.
Sensory properties
Although enzymatic cleavage of proteins is desirable for enhanced functionality in protein hydrolysates, the disadvantage associated with this mechanism is the bitterness development. The mechanism of bitterness has been documented to be linked with the presence of bitter peptides mostly comprising of hydrophobic amino acids. In addition, the native protein, the peptide sequences, the hydrolytic conditions, extent of hydrolysis, concentration and position of bitter taste residues, number of carbons on the ‘R’ group of branched chain amino acids and amino acid conformation are also influential in bitterness generation in peptides.
ROLE OF FPH IN AQUACULTURE PRODUCTION
Feed intake and utilization
The enzymatic hydrolysis process helps to formation of peptides with small molecular weight that may act as an attractant for fish. The availability of small molecular weight peptides and free amino acids in hydrolysed protein can improve the feed palatability which may stimulate feed intake of fish. The low molecular weight fractions of FPH reducing gluconeogenesis can increase the amino acids utilization of fish. The nutrient digestibility of fish increases with dietary inclusion of FPH with small molecular weight fractions in diets. Hence, the higher inclusion of FPH may negatively influence the digestibility in fish.
Growth performance
In order to the higher palatability and suitable peptide fractions (<10 kDa) produced from enzymatic process and molecular weight profile that improved the growth performance fish when fishmeal replaced by FPH. The replacement at 5-10 % of fishmeal with FPH improving growth performance of yellow croaker, Asian seabass and South American catfish juveniles. However, fish fed with higher FPH (≥20%) diets have been reported to decrease growth performance (Xu et al 2016; Siddik et al. 2018a). The higher hydrolysate levels leading to reduction in the growth performance may be due to an excessive number of short-chain peptides and free amino acids in these hydrolysed products, which could cause saturation of the peptide transport mechanism in the intestine. Also, higher amount of free amino acids could alter the absorption of amino acids leading to an increase of amino acid oxidation and reduced retention of dietary protein.
![]() Biochemical responses
Biochemical responses
The replacement of low FM diet by FPH led to an increase in haematocrit, haemoglobin, total protein and cholesterol levels, and decrease level of plasma glucose and triglyceride levels may indicate that the dietary inclusion of FPH leads to better absorption of the hydrolysed protein and enhancement of the general health condition of fish. Dietary inclusion of FPH in fish diets may trigger the innate immune system and disease resistance of fish. Immunoglobulin M, lysozyme activity and complement C4 were significantly higher in fish fed with diets containing 10% and 15% FPH when compared to fish fed with the basal diet (Tang et al., 2008; Siddik et al., 2019b). The low molecular weight bioactive peptides in FPH may have immune stimulating and antibacterial property.
CONCLUSION
Consider the amount of by-products produced from fish processing industries every year. FPH is one of these by-products which has potential effects on growth and biochemical responses in aquaculture due to their strong functional and bioactive characteristics. Hence, further research needs to develop the hydrolysis process without rendering the characteristics of the FPH.
References
1. Bhaskar, N., Benila, T., Radha, C., & Lalitha, R. G. (2008). Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresource technology, 99(2), 335-343
2. Chalamaiah, M., Rao, G. N., Rao, D. G., & Jyothirmayi, T. (2010). Protein hydrolysates from meriga (Cirrhinus mrigala) egg and evaluation of their functional properties. Food Chemistry, 120(3), 652-657.
3. Elavarasan, K. (2019). Health Benefits and Potential Applications of Fish Protein Hydrolysate. ICAR-Central Institute of Fisheries Technology.
4. Kristinsson, H. G., & Rasco, B. A. (2000). Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. Journal of agricultural and food chemistry, 48(3), 657-666.
5. Marchbank T, Limdi JK, Mahmood A, Elia G, Playford RJ. Clinical trial: protective effect of a commercial fish protein hydrolysate against indomethacin (NSAID)‐induced small intestinal injury. Aliment. Pharmacol. Ther 2008; 28:799-804.
6. Siddik MAB, Howieson J, Fotedar R (2019b) Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish and Shellfish Immunology 89: 61–70.
7. Siddik MAB, Howieson J, Ilham I, Fotedar R (2018a) Growth, biochemical response and liver health of juvenile barramundi (Lates calcarifer) fed fermented and nonfermented tuna hydrolysate as fishmeal protein replacement ingredients. PeerJ 6: e4870.
8. Tang HG, Wu TX, Zhao ZY, Pan XD (2008) Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). Journal of Zhejiang University Science B 9: 684–690
9. Xu H, Mu Y, Zhang Y, Li J, Liang M, Zheng K et al. (2016) Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): effects on growth performance and lipid accumulation. Aquaculture 454: 140– 147.