The shrimp industry is facing several ongoing challenges, including disease outbreaks caused by bacteria, as well as viruses and parasites like acute hepatopancreatic necrosis disease (AHPND/EMS), white feces disease, white spot virus (WSV), and EHP (Enterocytozoon hepatopenaei) infections. Indiscriminate, or illegal, use of antibiotics on the farm level has rendered many antibiotics useless. Now, farmers are left with few tools to combat these challenges. In response to this need, innovative feed additives are being called upon to help.

Global Category Manager Aquaculture
Agrimprove
Medium-chain fatty acids (MCFAs), with their successes in mitigating diseases and supporting antibiotic bans in terrestrial animals, have emerged as a potential new tool in the management of shrimp diseases. Medium-chain fatty acids are defined as saturated fatty acids with carbon chains of 6 to 12 atoms long and consist of caproic (C6), caprylic (C8), capric (C10), and lauric (C12) acids. These fatty acids occur naturally as triglyceride structures found in various feed ingredients. Agrimprove has a strong, scientifically backed, history in transforming these triglycerides into free fatty acids to produce proprietary combinations of MCFAs for different species including pigs, poultry, cattle, salmon, and shrimp.
The unique chemical structure determines the specific biological functions of the MCFA. These functions include inhibitory activity on the gram-negative bacteria vibrio (the root cause of many shrimp diseases), antimicrobial activity against both bacterial and viral pathogens, reducing pathogen virulence, improving intestinal morphology, and minimizing injury to hepatopancreas. With MCFAs known biological functions on land animals, several studies were performed to evaluate the effectiveness of MCFAs in shrimp. The summaries provided below indicate that MCFA blends, like Aromabiotic® Aqua, can help reduce or prevent dependence on antibiotics and produce healthier shrimp.
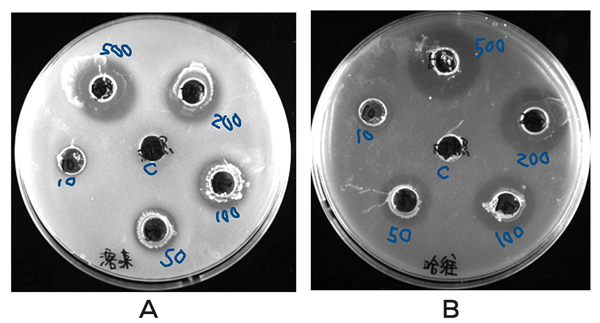
MCFA IN VITRO TESTS ON THE INHIBITORY ACTIVITY OF VIBRIO
Agar plates were prepared with V. alginolyticus or V. harveyi (each at 3 x 108 CFU/ml) at a 7.2 pH. Six holes were created and were filled with 10, 50, 100, 200, and 500 mg/ml MCFAs respectively, while the center hole filled with a reagent without MCFA as a control. Clear rings (inhibition zones) were observed in all concentrations. Respective to the doses, the radii of clear rings were 6, 8, 10, 12, and 15 mm for V. alginolyticus and 7, 8, 12, 14, and 16 mm for V. harveyi (Figure 1).
The bacteriostatic effect of the MCFAs on V. parahaemolyticus was also evaluated with a standard minimal inhibition concentration (MIC) test. On a medium with a pH adjusted to 7.0, the optical density (OD) of bacterial cultures were incubated for 24 hours. Different concentrations of the MCFA were then measured. At a concentration of 0.1%, the OD was statistically similar to the blank control. Therefore, this concentration can be considered as the minimal inhibition concentration for V. parahaemolyticus.
ANTIMICROBIAL ACTIVITY AGAINST BACTERIAL AND VIRAL PATHOGENS
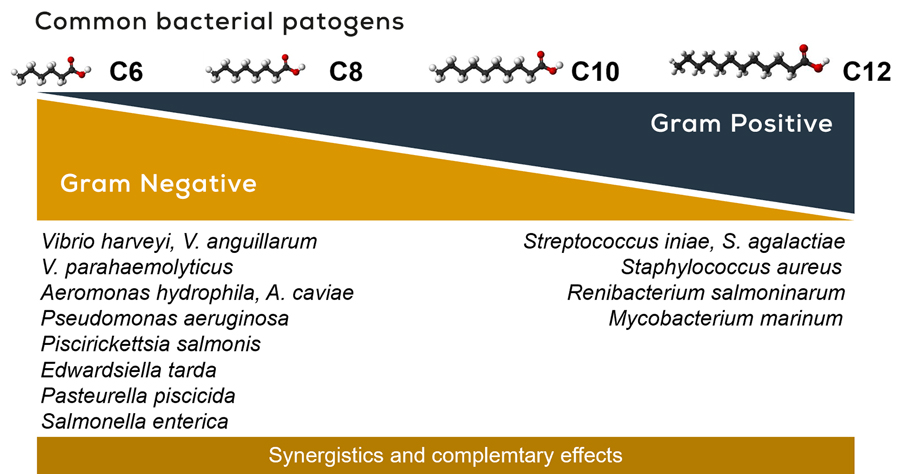
The MCFAs C6, C8, C10, and C12 were tested on common aquatic bacterial pathogens and found to complement each other and work synergistically to inhibit bacterial growth. It was found that C8 and C10 are more effective against gram-negative bacteria—such as Vibrio, Aeromonas, and Edwardsiella—while C10 and C12 are more effective against gram-positive bacteria like Streptococcus and Staphylococcus (Figure 2).
COMMON AQUATIC BACTERIAL PATHOGENS
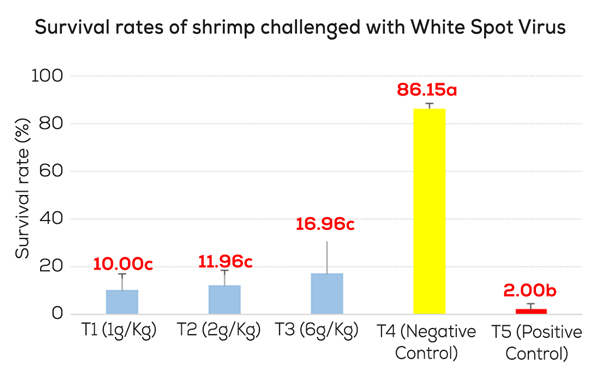
In one challenge trial conducted in Vietnam, Pacific white shrimp (Litopenaeus vannamei) were fed 0, 2, or 6 g/kg of MCFA for 14 days and then challenged with WSV (oral, 108 copies/g, 4 meals/day, at 5% BW). The survival rates (SR) were recorded for the next 10 days. The negative control (no virus, no MCFA) had a 86% SR, the positive control (with virus, no MCFA) has 2% SR, and the MCFA groups (with virus and MCFA) have 12 and 17% SR (Figure 3).
Reducing pathogen virulence
Bacteria secrete two enzymes, caseinase and haemolysin, that are specifically designed to enter and damage the host’s tissues and cells. The activity of these enzymes is therefore directly linked to virulence/damage to the host. In a trial, MCFAs at concentrations of 0.025 and 0.05% lowered the caseinase activity of V. harveyi BB120, but there was no effect on haemolysin activity. Therefore, by lowering the caseinase activity, MCFAs are shown to reduce pathogen virulence and specifically can render vibrio less infectious.
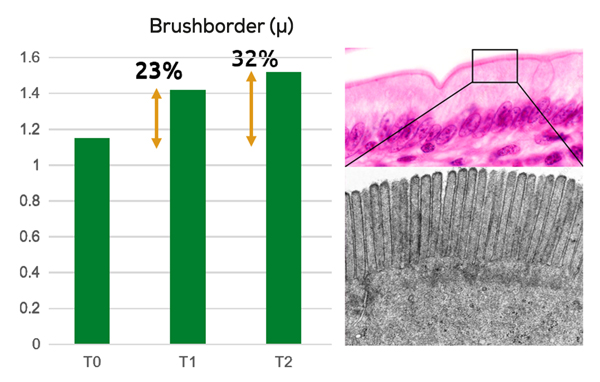
Improving intestinal and hepatopancreatic morphology
Pacific white shrimp (L. vannamei) were fed 0, 4 and 4.5 g/kg of MCFA for two weeks before the health of the intestine morphology was evaluated by measuring the height of the brush border. The MCFAs increased the brush border height by 23% and 32% comparing to the control (Figure 4). It is understood that the higher the brush border height, the larger the surface area, and the better the absorption of nutrients.
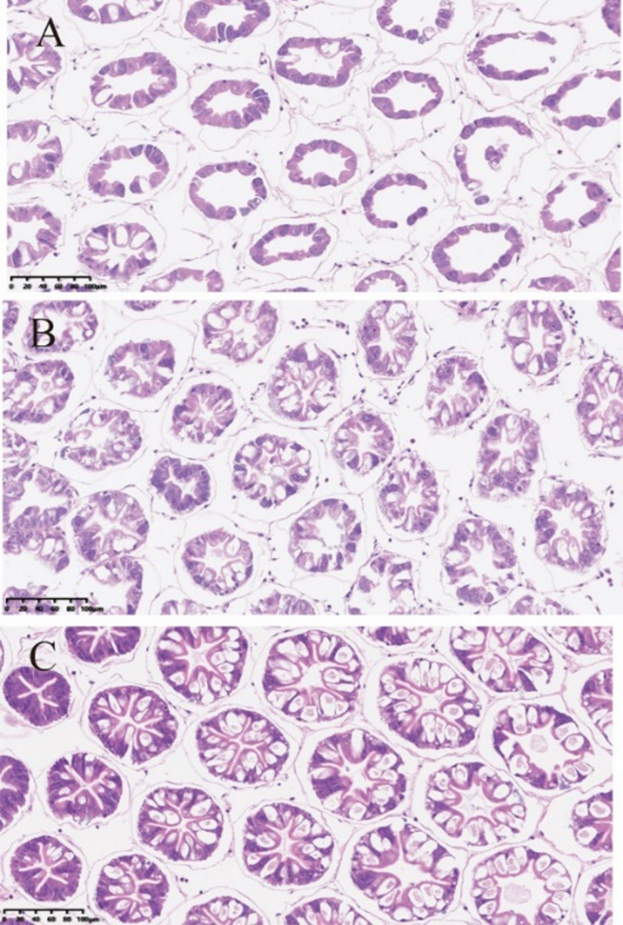
Pacific white shrimp (L. vannamei) were fed 0, 1, and 5 g/kg of MCFA for 5 weeks. At the end of the trial, the histological structure of hepatopancreas in the MCFA groups was improved compared with the control. It was found the arrangement of the hepatopancreas was more compact, the hepatic corpuscles were fuller and denser, the lumen was star-shaped, the basement membrane was intact, and the structure of star-shaped lumen were more obvious in MCFA groups (Figure 5).
Minimizing injury to hepatopancreas
Similar to the liver of vertebrates, the shrimp’s hepatopancreas is the main metabolic organ and detox center of the animal. Aspartate transaminase (AST) and alanine aminotransferase (ALT) are known indicators that reflect injuries in the hepatopancreas. Shrimp fed MCFAs were found to have decreased ALT and AST activity in their muscles, which may reflect the improved hepatopancreatic health.
MCFAs have a higher pKa (4.90–5.00) than all the short-chain fatty acids (SCFA). The high pKa increases MCFAs efficacy in acidic environments like the stomach of a shrimp (pH 5.7) and allows for synergistic effects with pH-reducing acids (fumaric acid, lactic acid, butyric acid, etc.). The mode of action of MCFAs against pathogenic bacteria can be summarized in 3 steps:
1. Creating pores in the bacteria membrane through the lipophilic and hydrophilic ends of MCFA
2. Once through the membrane and inside the bacterial cell, MCFAs dissociate and acidify the cell’s contents to disturb cell metabolism and protein synthesis
3. Blocking bacterial DNA replication through DNA intercalation and stopping cell divisions.
All these lead to bacterial cell death.
Similarly, for viruses, especially enveloped viruses like WSV—where the envelope is made of a lipid bilayer—MCFAs do the same as they do to the bacteria membrane; create pores in the membrane, rupture the membrane, and kill the virus.
MCFAs are useful in preventing and mitigating bacterial and viral disease challenges faced by shrimp farmers. Growing evidence shows that it can be a new tool for farmers, reducing reliance on antibiotics and securing a good production yield. For disease prevention, the dose range is typically 0.1 to 0.3% of the feed. For therapeutic use, the dose range is 0.5 to 1.0%. This also depends on the concentration of the MCFA as well as if it is incorporated into the feed by the feed mills or top-coated at the farm sites. When top-coated, higher doses are normally required to counter possible leaching to pond water.
About Fuci Guo
Fuci Guo joined Royal Agrifirm Group as Global Category Manager – Aquaculture in October 2021. His objective is to replicate the successes of Agrimprove’s feed additives in terrestrial animals to aquatic species, especially shrimp. He has an Aquaculture degree from China Ocean University, a Masters from the National University of Singapore, and a Ph.D. from the University of Guelph, Canada. With strong network connections to both feed mills and farmers, Guo brings to Agrifirm over 25 years of experience in aquaculture nutrition and health from various multinational corporations.